ADVANCED TOPICS IN LASER SPECTROSCOPY I PHYS 286
Fall 2002 Homework #1 Dr. P. Misra
Due 09/09/02
1. Use the Boltzmann distribution to calculate the numbers of molecules in the v=1 state compared to the number in v=0 state at 25°C for Br2, which has the rather small vibrational energy-level spacing of 323 cm-1, and for H2, which has a vibrational spacing of 4395 cm-1, the largest for any molecule.
2. Calculate the moments of inertia of HCl35, HCl37 and DCl35, all of which have an equilibrium bond length of 1.275 Å. Estimate the energy difference between the lowest and first excited rotational states for each molecule.
3. The zero-point vibrational energy for H2 is 0.265 eV. Compare the vibrational energy of H2, D2 and HD numerically for the low-lying states.
4. The potential energies of two diatomic molecules of the same reduced mass are shown in the figure below:
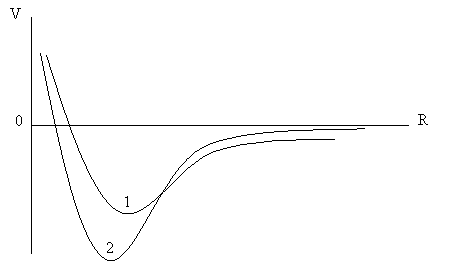
From the above graph determine (giving suitable reasons for your choice) which molecule has the larger:
(a) internuclear distance
(b) rotational moment of inertia
(c) separation between rotational energy levels of the same J and V
(d) binding energy
(e) zero-point energy
(f) separation between low-lying vibrational states.