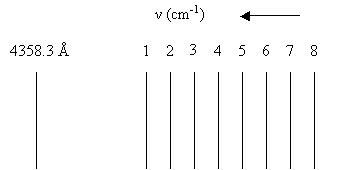|
ADVANCED
TOPICS IN LASER SPECTROSCOPY I PHYS
286 |
||
|
Fall
2002 |
Homework
#3 |
Dr.
P. Misra |
|
1. (a) In the pure rotational Raman spectrum of homonuclear diatomic molecule a 5:3 intensity alternation is observed. Assuming that the portion of the wave function exclusive of nuclear spin and rotation is totally symmetric, explain the observed intensity alternation with the aid of a well-labeled energy level diagram. (b) For a certain diatomic molecule, the fundamental vibration-rotation band in the ground electronic state occurs at 4 μm in the infrared. The raman spectrum for the same molecule was excited by using the radiation from a certain source; where the filter between the source and the sample transmits the monochromatic wavelength 4000 Å. Calculate (to the nearest Å) the wavelength of the Raman band that one would observe corresponding to the infrared fundamental. |
||
|
2. In a portion of the solar spectrum, a group of four lines were identified to be due to absorption by the (1-0) band of 14N2 in the Earth’s atmosphere. The wavenumbers of these lines (in cm-1) were: 2395.96, 2403.57, 2411.13, and 2418.65. Identify the S-branch transitions (ΔJ = +2) responsible for these four lines on a clearly labeled energy-level diagram. (Remember that N2 is a homonuclear diatomic molecule and has no electric dipole transitions corresponding to ΔJ = 0; but it does have electric quadrupole allowed transitions corresponding to ΔJ = +2). |
||
|
3. A portion of the Raman spectrum of 14N2 corresponding to the Stokes branch is excited using the 4358.3 Å line of mercury.
The wavenumbers (in cm-1) of the Raman lines with serial numbers are: 22,908.92, 22,900.96, 22,893.01, 22,885.06, 22,877.11, 22,869.16, 22,861.22, and 22,853.28, respectively. Determine the J assignments for the eight Raman lines. |
||