|
STATISTICAL MECHANICS PHYS 222 |
||
|
Fall 2002 |
Homework #3 Due 09/18/02 |
Dr. P. Misra |
|
1.
Define |
||
|
2. We wish to calculate vapor pressure of water at 20°C more accurately. (a) Heat capacity of water is 1 cal/(g°C). Heat capacity of water vapor (steam) is 0.35 cal/(g°C). What is the latent heat at 20°C? (b) Use the average latent heat to calculate the vapor pressure of water at 20°C. |
||
|
3.
Assuming constant CV, calculate the
entropy S of one mole of Van der Waals gas for a given volume V
and temperature T. Show that
your result is identical to the eqn. |
||
|
4.
Assuming constant CV, what is the equation
for the PV-curve for the adiabatic process of a van der Waals gas? Show that your result is identical to the
eqn.
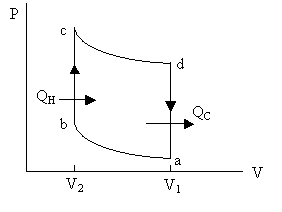
Fig. 1. Otto cycle for automobile engine. ab and cd are adiabatic processes. bc and da are constant volume processes. QH is the heat from the
burning gasoline. QC
is the waste heat out of the exhaust pipe.
(H=hot, C=cold) |
||
|
5. The automobile gasoline engine can be approximated by the Otto cycle shown as Fig. 1. For the intake and compression strokes ab, air-gasoline mixture is compressed adiabatically from V1 to V2. For the power stroke, gasoline burns to produce energy QH (bc at constant volume V2) and then expands adiabatically (along the path cd) from V2 to V1. For the exhaust stroke da, waste gas and the waste heat QC goes out of the tailpipe at the constant volume V2. The compression ratio R of the engine is defined as R = V2/V1. Assuming ideal gas behavior, show that the efficiency of the engine is (QH – QC) / QH = 1–Rg-1, where g = CP/CV is defined by the eqn. g = 1+(R/CV) and R < 1. |
||
 .
.