|
STATISTICAL MECHANICS PHYS 222 |
||
|
Spring 2002 |
Homework #4 Due 09/20/02 |
Dr. P. Misra |
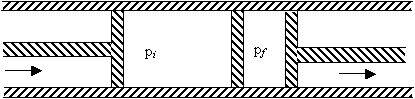
(a) By considering the work done by the gas in the above throttling process, find the initial and final enthalpies of the gas and the relationship between them. (b) What can be said about the intermediate states of the system? (c) Calculate the temperature difference (ΔT) between the two regions for a small pressure difference Δp = pf - pi. (d) Using the above result, discuss the possibility of using the process to cool either an ideal gas, or a more realistic gas obeying the equation p (V-b) = RT. Explain your result. |
||
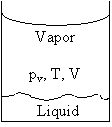
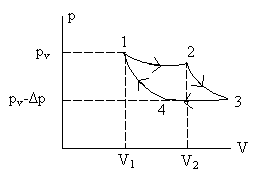
The thermodynamic cycle progresses isothermally from state 1 to state 2, evaporating n moles of liquid. This is followed by reversible cooling from 2 to 3, and subsequently a contraction from 3 to 4, which results in condensation of n moles of liquid. Finally, a reversible heating of the system from 4 to 1 completes the cycle. (a) Noting that V2 – V1 = Vg – Vl, where Vg = volume of n moles of gas and Vl = volume of n moles of liquid, calculate the efficiency in terms of Δp, Vg – Vl, and the latent heat Lv of vaporization of a mole of liquid. (b) Use the result of part (a) to obtain an expression for dpv/dT in terms of (Vg – Vl), n, Lv and T. |
||